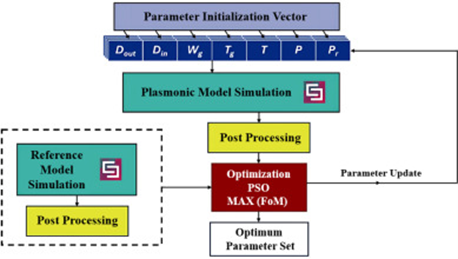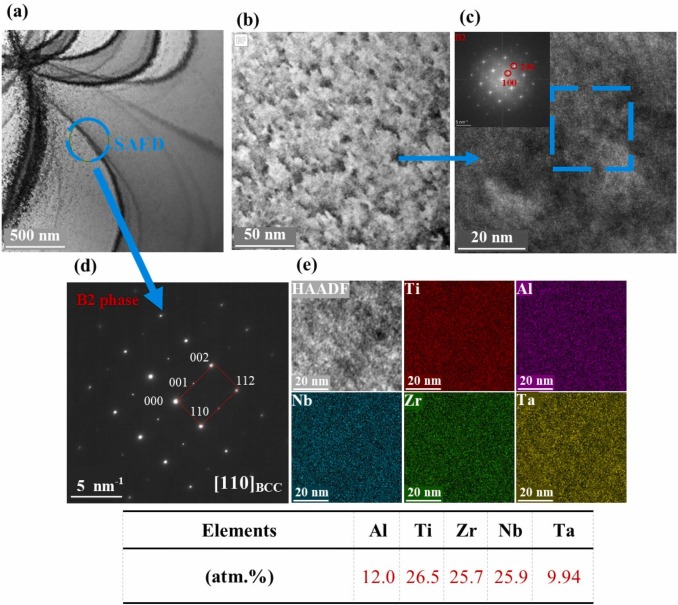
Caspase-4/11 exacerbates disease severity in SARS–CoV-2 infection by promoting inflammation and immunothrombosis
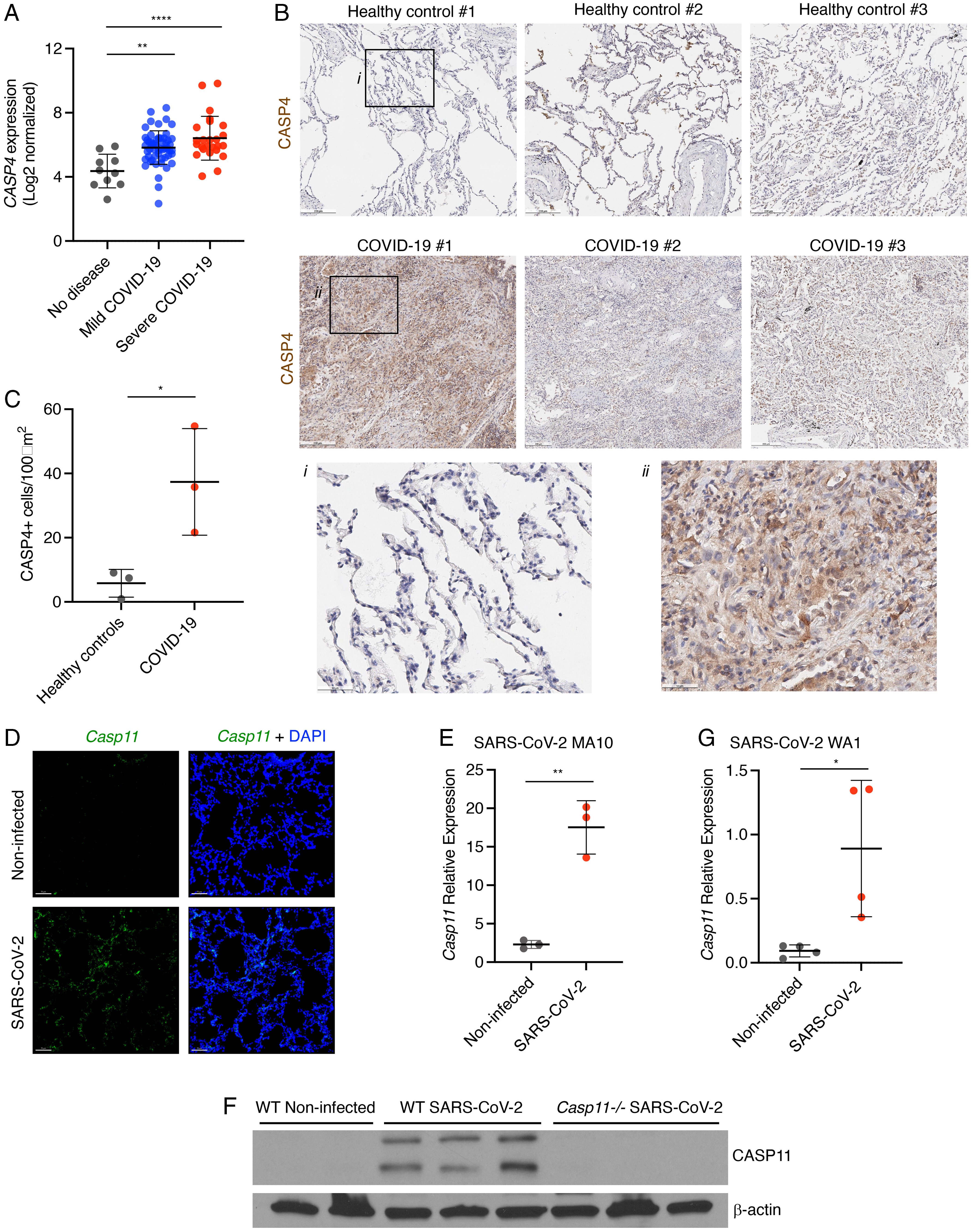
Severe acute respiratory syndrome coronavirus 2 (SARS–CoV-2) is a worldwide health concern, and new treatment strategies are needed. Targeting inflammatory innate immunity pathways holds therapeutic promise, but effective molecular targets remain elusive. Here, we show that human caspase-4 (CASP4) and its mouse homolog, caspase-11 (CASP11), are up-regulated in SARS–CoV-2 infections and that CASP4 expression correlates with severity of SARS–CoV-2 infection in humans. SARS–CoV-2–infected Casp112/2 mice were protected from severe weight loss and lung pathology, including blood vessel damage, compared to wild-type (WT) mice and mice lacking the caspase downstream effector gasdermin-D (Gsdmd2/2). Notably, viral titers were similar regardless of CASP11 knockout. Global transcriptomics of SARS–CoV-2–infected WT, Casp112/2, and Gsdmd2/2 lungs identified restrained expression of inflammatory molecules and altered neutrophil gene signatures in Casp112/2 mice. We confirmed that protein levels of inflammatory mediators interleukin (IL)-1β, IL-6, and CXCL1, as well as neutrophil functions, were reduced in Casp112/2 lungs. Additionally, Casp112/2 lungs accumulated less von Willebrand factor, a marker for endothelial damage, but expressed more Kruppel-Like Factor 2, a transcription factor that maintains vascular integrity. Overall, our results demonstrate that CASP4/11 promotes detrimental SARS–CoV-2–induced inflammation and coagulopathy, largely independently of GSDMD, identifying CASP4/11 as a promising drug target for treatment and prevention of severe COVID-19. Copyright © 2022 the Author(s).