Advanced materials and technologies for supercapacitors used in energy conversion and storage: a review
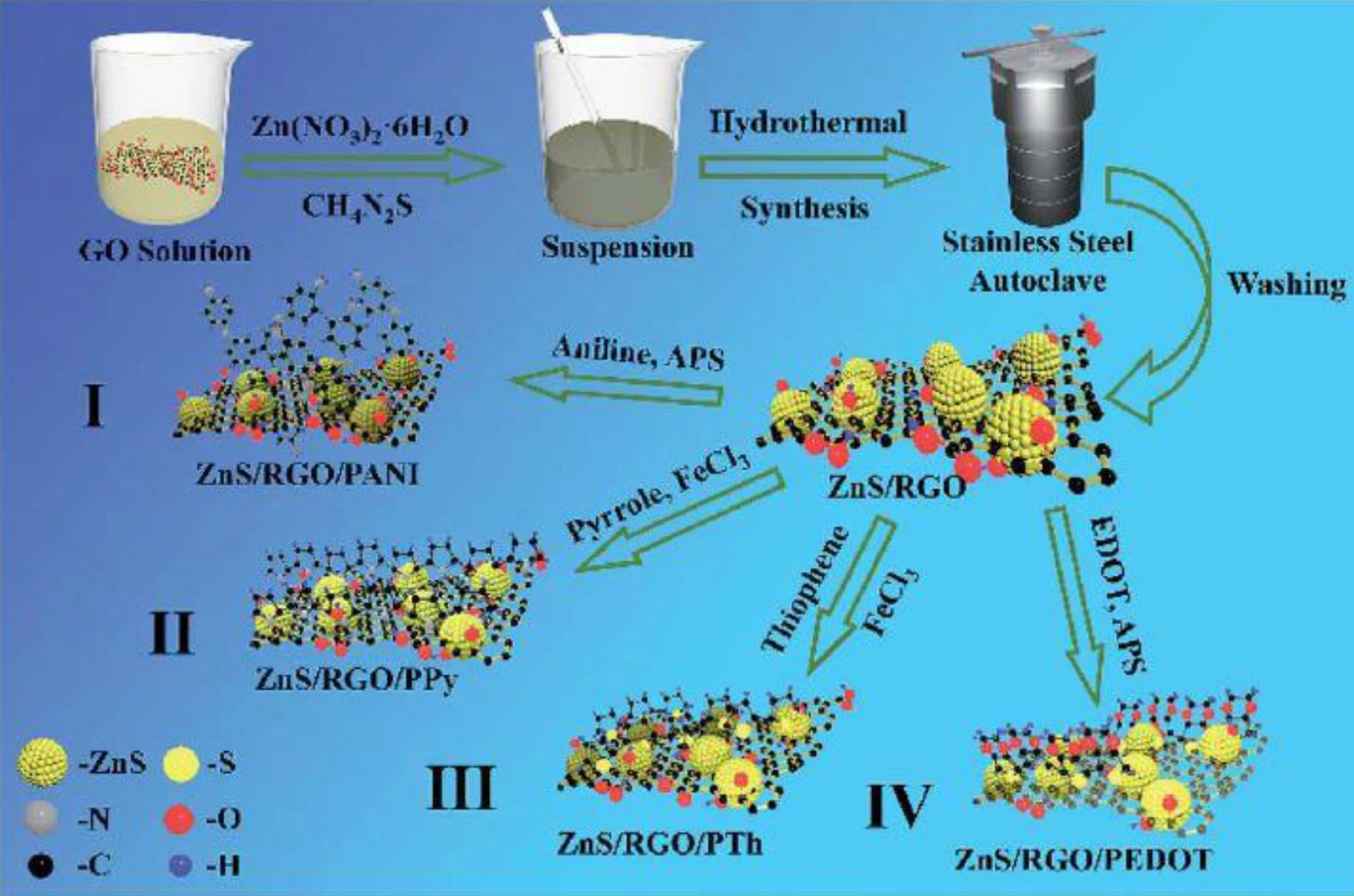
Supercapacitors are increasingly used for energy conversion and storage systems in sustainable nanotechnologies. Graphite is a conventional electrode utilized in Li-ion-based batteries, yet its specific capacitance of 372 mA h g−1 is not adequate for supercapacitor applications. Interest in supercapacitors is due to their high-energy capacity, storage for a shorter period and longer lifetime. This review compares the following materials used to fabricate supercapacitors: spinel ferrites, e.g., MFe2O4, MMoO4 and MCo2O4 where M denotes a transition metal ion; perovskite oxides; transition metals sulfides; carbon materials; and conducting polymers. The application window of perovskite can be controlled by cations in sublattice sites. Cations increase the specific capacitance because cations possess large orbital valence electrons which grow the oxygen vacancies. Electrodes made of transition metal sulfides, e.g., ZnCo2S4, display a high specific capacitance of 1269 F g−1, which is four times higher than those of transition metals oxides, e.g., Zn–Co ferrite, of 296 F g−1. This is explained by the low charge-transfer resistance and the high ion diffusion rate of transition metals sulfides. Composites made of magnetic oxides or transition metal sulfides with conducting polymers or carbon materials have the highest capacitance activity and cyclic stability. This is attributed to oxygen and sulfur active sites which foster electrolyte penetration during cycling, and, in turn, create new active sites. © 2020, The Author(s).